The Curious Case of the Smelly Blob
- Nov 5, 2020
- 0 Comments


I’m not too fond of unsolved mysteries. I never have. So, when a service contractor stumped us with a problem in the late 1990s, I couldn’t shake it.
The contractor told us about gelatinous masses building up in a handful of grease separators. The masses smelled terrible, he said, and the separators had surprisingly low water input flows.
We tried figuring out what the gel was. We ran tests in our lab. We looked online. We consulted wastewater treatment professionals and scientists. We just couldn’t come up with anything.
I kept picturing the monster from the 1958 cinematic classic, The Blob. Reluctantly, we filed the data and moved on.

Fast forward to 2011, when we learned of a major donut chain site in Beaverton, Or., where a concrete grease interceptor was literally falling apart after only six years. Beaverton’s Public Works Department manages its sewer collection system. Its collection flows are treated by the regional treatment authority, Clean Water Services. For years, the city had been jetting the collection line downstream of the donut store anytime CCTV assessment showed build-up in the line. There was always build-up in the collection line.
We cut a deal with the franchisee. Thermaco paid to install a Trapzilla TZ-600 separator on site. The franchisee let us have an independent research firm, Environmental Engineering & Contracting (EEC), conduct a comprehensive before-and-after study of fats, oils, and grease (FOG), total suspended solids (TSS), pH levels, and biological oxygen demand (BOD).
EEC collected several weeks’ worth of effluent samples from the concrete grease separator and, later, from the Trapzilla separator. The results shocked us. The study showed the site had virtually no grease in the effluent.
Why, then, was there so much “grease” in the downstream line? Why was the pH extremely low? Why was the FOG averaging 6674 mg/l downstream of the concrete separator and nearly 2/3 less after the Trapzilla separator was installed? Why did the TSS increase with the installation of the Trapzilla?
It was another mystery that needed solving.
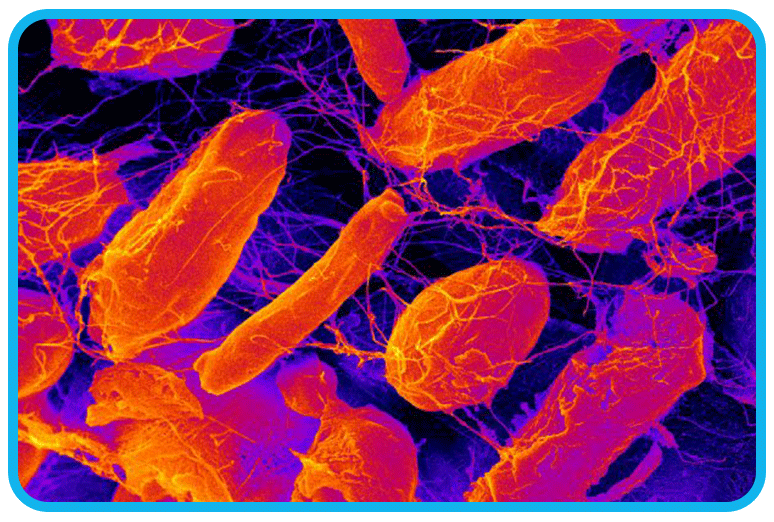
This time, however, we had more data to work with. We discovered a specific bacterium, Acetobacter Xylinum, was eating the sugar, flour, and yeast in the pipes from the donut operation.
Acetobacter xylinum (also referred to as "Komagataeibacter xylinus") love donuts as much as we do. Humans have a multi-stage digestive tract to break down complex foods in a matter of hours. Bacteria, on the other hand, emit enzymes to digest food. A bacterium will also reproduce quickly and release toxins to block competing bacteria from the food source.
In Beaverton, Acetobacter xylinum produced acetic acid because other bacteria could not withstand a pH below 5.0. It also protected its offspring by building dams and domiciles made of cellulose. You read it correctly – cellulose, the same stuff in cotton and wood.
I flashed back to the 1990s call with the service contractor and realized that smelly blob he reported had to be cellulose. The terrible odor he described resulted from low pH leading to any sulfur compounds immediately transitioning to sulfide. Another mystery solved!
Acetobacter xylinum likes low-flow conditions where it can concentrate its acidic pH advantage over other bacteria wanting in on its food source. Acetobacter xylinum prefers alcohols, so it loves sites with sugars and yeasts for converting starches into alcohols. (It is also the reason an opened bottle of wine tastes like vinegar after a few days.)
For our chemist readers, note that ethyl alcohol (the alcohol in beer, wine, and liquors) is CH2H5OH. Vinegar (acetic acid) is CH2H5OOH. Only one oxygen difference between ethyl alcohol and acetic acid and Acetobacter Xylinum takes ethyl alcohol and rapidly makes acetic acid to shoo away its competitors.
Ok, you say. We understand the low pH and cellulose gunk creation story. What about the significant changes seen in the BOD and TSS between the two different separators?
The 1000-gallon concrete grease interceptor had water turnover every 5 to 7 days. The site used about 100 gallons of water per day for kitchen and utensil cleaning operations. The Trapzilla unit’s 95-gallon capacity saw daily water turnover. Carbohydrates in the kitchen drainage were flour, cane sugar, and milk sugars. Sugars were immediately available for yeast digestion, while flour took longer for yeast and bacterial digestion.
The larger separator's 7-day water turnover gave plenty of time for the conversion processes and thus the lower pH and higher BOD. (Raw flour does not fully show its full BOD value on a BOD 5-day test.) The longer holding time in the 1000-gallon tank “processed” the raw flour into constituents more amenable to being eaten by the bacterial of the BOD 5-day test.
The longer holding period also made more alcohol production from the sugars and flour carbohydrates, resulting in much lower pH values. The same quantity of flour was present for both separators. The smaller separator’s daily water turnover had a higher TSS because these particles were undigested. No/less digestion in the smaller separator explains the higher (more favorable) pH values, the significantly lower BOD (undigested carbohydrates in the BOD 5-day test), and higher TSS (flour particles did not experience seven days of digestion by yeast and subsequently acetobacter xylinum bacteria).
Here's a simpler explanation. Low flow, long retention time, and lots of sugars/carbohydrates lead to low pH conditions caused by happy Acetobacter Xylinum bacteria. You smell their actions in brewery drains (alcohols, yeasts, carbohydrates and low flows) and coffee shop drains (sugars, shallow flows), and other sites having those flow and foods characteristics.
Don’t get stumped. When you find stuff that looks like grease, stinks especially badly, and the site has little or no F.O.G. in its effluent, it’s no mystery. It’s probably Acetobacter xylinum.
Share:
Posted in FOG Pretreatment